The Molecular Structure of Water Is Best Described as __________
Each molecule has both positive and negative poles because the positive and negative changes are unevenly distributed. The bonds are highly polar.
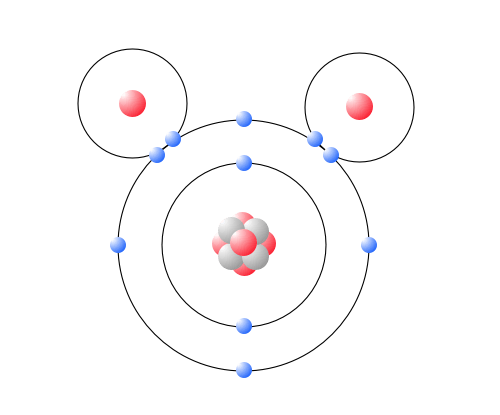
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation
The atoms in the molecule of water share electrons.

. How will you describe the molecular structure of water. Structure of water molecule is made up of one molecule of oxygen and two molecules of hydrogen bonded covalently. A water molecule is made up of two hydrogen atoms and one oxygen atom.
The oxygen atom is s p 3 hybridized which results in tetrahedral electron pair geometry and bent or V shaped molecular geometry. How do the atoms interact. The electronegativity difference between oxygen and hydrogen is large.
When two hydrogen atoms are bound to an oxygen atom the outer electron shell of oxygen is filled. 1 Oxygen atom. The chemical formula for this is H20 with the 2 in theformula small at the bottom of the H.
The option D describe the molecular structure of water. This imparts partial negative charge to the oxygen atom and partial positive charge to hydrogen atoms. In other words it has two atoms of Hydrogen H and one of OxygenO.
The molecular structure of water is H₂O. Water H 2 O essentially considered one of the most important substances found on the earth. Every molecule of water is made of.
A single oxygen atom contains six electrons in its outer shell which can hold a total of eight electrons. A water molecule consists of one oxygen atom bonded to two hydrogen atoms by covalent bonds. Water is a liquid consisting of 2 parts hydrogen and one partoxygen.
The diagram represents a water moleculeThis molecule is best described as polar with polar covalent bonds A substance that has a melting point of 1074 K conducts electricity when dissolved in water but does not conduct electricity in the solid phase. Oxygen atoms have partial negative charge while hydrogen atoms have partial positive charge. It covers over 70 of the earths surface and makes up as much as 95 of the living organisms.
The water molecule formed through covalent bonding is known as a polar molecule. Water basic chemical structure is best described as - 24440692 lakotaskyloseth lakotaskyloseth 4 weeks ago Biology College answered expert verified Water basic chemical structure is best described as 2 See answers Advertisement. Water is a tiny bent molecule with the molecular formula H 2 O consisting of two light hydrogen atoms attached to each 16-fold heavier oxygen atom.
Describe the molecular structure of water and explain why it is a polar molecule. Since oxygen is more electronegative as compared to hydrogen atoms the shared electrons are attracted towards the oxygen atom. As a physics student and not a chemist I think to myself Okay there are two lone pairs they will repel each other and.
The O atom has two bond pairs of electrons and two lone pairs of electrons. Water has the formula ceH2O and we can draw a Lewis structure with two lone pairs on the central oxygen. Water H 2 O is the result of the covalent between a single oxygen atom and two hydrogen atoms.
Each molecule is electrically neutral but polar with the center of positive and negative charges located in different places. Water molecule is polar.

The Structure And Properties Of Water Introduction To Chemistry
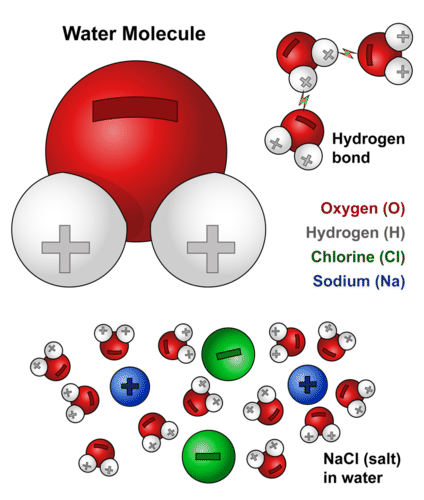
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation
Comments
Post a Comment